| NDC | 67225-0010-1 |
| Set ID | dde82f7d-e6bb-49af-a4c8-979821b7436f |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Aekyung Industrial Co., Ltd. |
| Generic Name | |
| Product Class | |
| Product Number | |
| Application Number | PART332 |
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
-
DOSAGE & ADMINISTRATION
Do not swallow. Rinse mouth with water after brushing.
Children under 6 years:
- To minimize swallowing, use a pea-sized amount. Supervise brushing until good habits are established.
- If swallowed more than used for brushing, seek professional assistance or contact a Poison Control Center immediately.
- INDICATIONS & USAGE
- INSTRUCTIONS FOR USE
- DO NOT USE
- STORAGE AND HANDLING
- QUESTIONS
- SPL UNCLASSIFIED SECTION
-
INACTIVE INGREDIENT
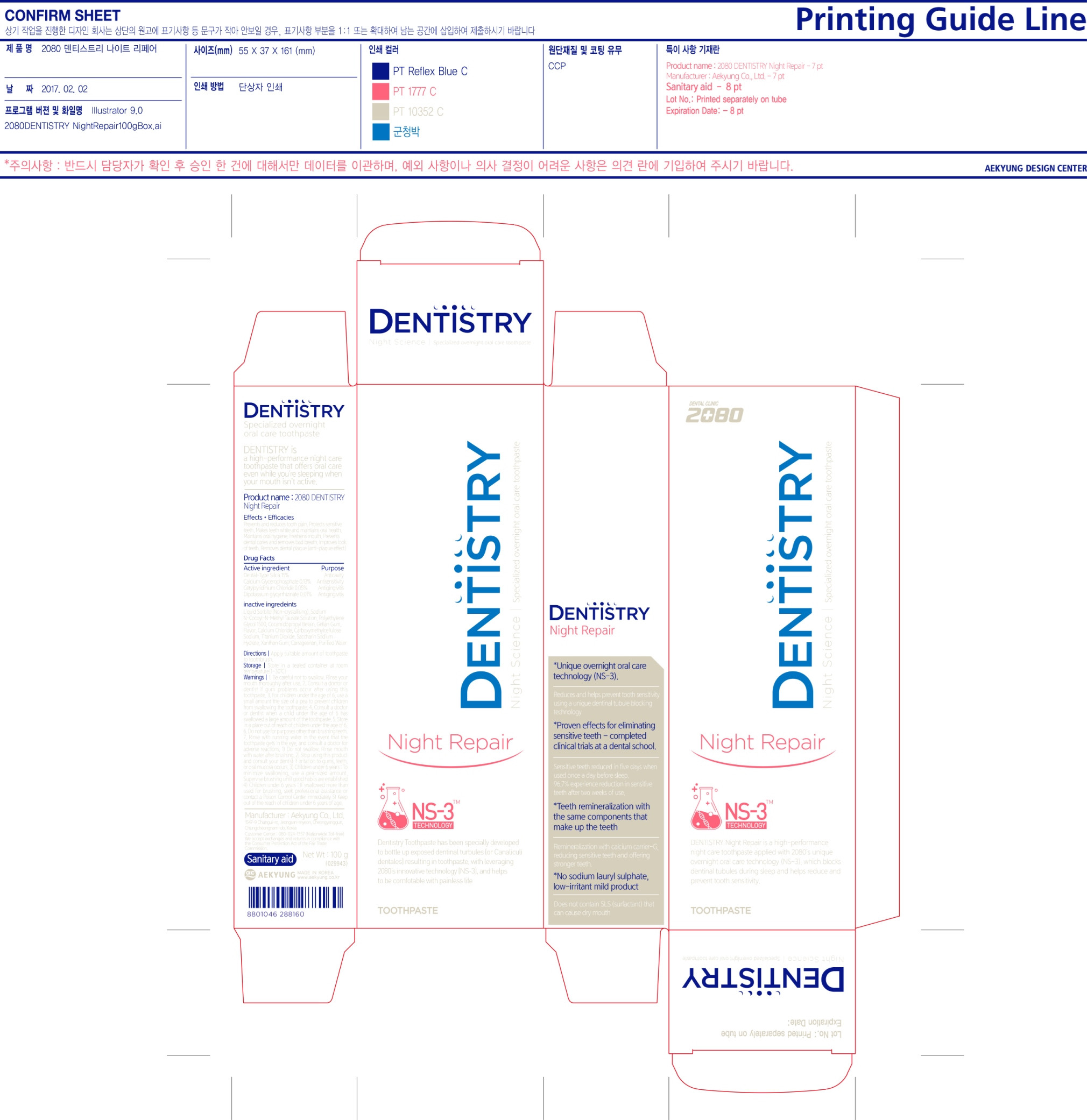
Inactive Ingredients: Liquid Sorbitol (Non-crystalizing), Sodium N-Cocoyl-N-Methyl Taurate Solution, Polyethylene Glycol 1500, Cocamidopropyl Betain, Gellan Gum, Flavor, Calcium Chloride, Carboxymethylcellulose Sodium, Titanium Dioxide, Saccharin Sodium Hydrate, Xanthan Gum, Carrageenan, Purified Water
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
2080 DENTISTRY NIGHT REPAIR
dental type silica (silicon dioxide), calcium glycerophosphate, cetylpyridinium chloride, dipotassium glycyrrhizinate paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67225-0010 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 15 g in 100 g Inactive Ingredients Ingredient Name Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) CALCIUM GLYCEROPHOSPHATE (UNII: XWV9Z12C1C) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) SORBITOL (UNII: 506T60A25R) SODIUM METHYL COCOYL TAURATE (UNII: JVL98CG53G) POLYETHYLENE GLYCOL 1500 (UNII: 1212Z7S33A) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) SACCHARIN SODIUM (UNII: SB8ZUX40TY) GELLAN GUM (LOW ACYL) (UNII: 7593U09I4D) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) CARRAGEENAN (UNII: 5C69YCD2YJ) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67225-0010-1 1 in 1 CARTON 02/03/2017 1 100 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part332 02/03/2017 Labeler - Aekyung Industrial Co., Ltd. (687995647) Establishment Name Address ID/FEI Business Operations Aekyung Ind. Co., Ltd._Chungyang Factory 690511126 manufacture(67225-0010)