| NDC | 52124-0001-1, 52124-0003-1, 52124-0004-1, 52124-0005-1, 52124-0008-1, 52124-0009-1, 52124-0010-1, 52124-0011-1, 52124-0111-1 |
| Set ID | 7d1950b4-3237-4512-bab3-4c7364bdd618 |
| Category | HUMAN OTC DRUG LABEL |
| Packager | Genuine First Aid LLC |
| Generic Name | |
| Product Class | Amide Local Anesthetic, Aminoglycoside Antibacterial, Antiarrhythmic |
| Product Number | |
| Application Number | PART333 |
- ACTIVE INGREDIENT
- PURPOSE
Purpose: First aid antiseptic, external analgesic
Close
Uses: First aid to help prevent infection and for the temporary relief of pain and itching associated with:
Minor Cuts
Scrapes
Burns - WARNINGS
- DO NOT USE
Do not use: In eyes, in large quantities, over raw blistered areas, or on deep puncture wounds, animal bites or serious burns, for more than one week
Close - KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
Directions: Clean affected area, Apply small amount not more than 3 times daily.
Close
May be covered with a sterile bandage. - STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
Genuine First Aid Burn Cream
Close
Antiseptic Pain Relief With Aloe
Net Wt 0.9g (1/32 oz)
Manufactured in CHINA for
Genuine First Aid. - ACTIVE INGREDIENT
- PURPOSE
Use: For Professional and Hospital use. Helps prevent infection. Antiseptic cleansing of face, hands and body without soap and water.
Close - WARNINGS
- KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children: If swallowed, get medical help or contact a Poison Control Center right away.
Close - STOP USE
Stop use if unusual redness, swelling or other symptoms occur. Consult a physician immediately.
Close - DO NOT USE
- WHEN USING
Directions: Tear open packet, unfold towelette and use to cleanse desired skin area. Discard towelette appropriately after single use.
Close - INACTIVE INGREDIENT
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
Antiseptic Towelette
Close
Genuine First Aid LLC, Clearwater FL 33755
www.GenuineFirstAid.com
1/pouch
GENUINE FIRST AID - ACTIVE INGREDIENT
Active Ingredient: .........Bacitracin Zinc 400 units
Close
Neomycin Sulfate 5mg ( equivalent to 3.5 mg Neomycin base)
Polymyxin B Sulfate 5000 units - PURPOSE
- WARNINGS
- DO NOT USE
Do not use: in eyes; over large areas of the body;
Close
If allergic to any of the ingredients; for more than one week unless directed by a physician. - STOP USE
Stop use and consult a doctor:
Close
if the condition persists or gets worse; a rash or other allergic reaction develops - KEEP OUT OF REACH OF CHILDREN
- WHEN USING
Directions: clean affected area; apply small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily; may be covered with a sterile bandage
Close - STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
Genuine Triple Antibiotic
Close
First Aid Ointment
To Help Prevent Infection
Each Gram Contains:
Bacitracin Zinc 400 units
Neomycin Sulfate 5 mg
(equivalent to 3.5 mg
Neomycin base)
Polymyxin B Sulfate 5000 units
Net Wt. 0.5g ; (1/64 oz)
Manufactured in CHINA for
GENUINE FIRST AID. - ACTIVE INGREDIENT
- INACTIVE INGREDIENT
Inactive Ingredients:
Close
Sodium Chloride USP 44mg
Monobasic Sodium Phosphate USP 18mg
Sodium Phosphate Dibasic USP 111mg
Edetate Disodium USP 10mg
Benzalkonium Chloride 0.5mg
NF (as preservative) - STORAGE AND HANDLING
Store in a cool place. For irrigation only.
Close
Discard unused portion of the solution.
Not for injection. - WARNINGS
Warning:
Close
If you experience eye pain, changes in vision, continued redness or irritation of the eye,
or if the condition worsens or persists for more than 72 hours, discontinue use and consult a doctor.
Do not use if solution changes color or becomes cloudy. - DOSAGE & ADMINISTRATION
Close
Directions
Remove contacts before using.
Twist top to remove.
Flush the affected area as needed. Control
Rate of flow by pressure on the bottle. Do not touch
tip of the container to any surface. Do not reuse.
If necessary continue flushing with emergency eyewash or shower.
Discard bottle after use. - PURPOSE
Uses:
Close
For flushing or irrigating the eyes to
remove loose foreign material, air pollutants,
or chlorinated water. - DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
Active Ingredient: Purpose:
Close
Benzocaine, 6% w/v.................. Topical Anesthetic
SD alcohol, 60% w/v.................. Antiseptic - PURPOSE
Use: For the temporary relief of pain and itching associated with minor burns, scrapes and insect bites.
Close - WARNINGS
Warnings: For external use only.
Close
Avoid contact with eyes. If this happens, rinse thoroughly with water. - KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Close - STORAGE AND HANDLING
- DO NOT USE
Do not use: In eyes, on broken skin, deep puncture wounds. If unusual redness, swelling, irritation or other symptoms occur, consult a physician immediately.
Close - DESCRIPTION
- PRINCIPAL DISPLAY PANEL
Insect Sting Relief Pad
Close
Genuine First Aid LLC, Clearwater FL 33755
www.GenuineFirstAid.com
1/pouch
GENUINE FIRST AID - ACTIVE INGREDIENT
Active ingredient (in each tablet) Purpose
Close
Ibuprofen USP (NSAID*) 200mg . . . . . . . . . . . .Pain reliever/fever reducer
*nonsteroidal anti-inflammatory drug - PURPOSE
Close
Uses temporarily relieves minor aches and pains due to:
the common cold
headache
toothache
muscular aches
backache
minor pain of arthritis
menstrual cramps temporarily reduces fever - WARNINGS
Warnings
Close
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include: shock, facial swelling, asthma (wheezing) rash, skin reddening, blisters, hives If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause severe stomach
bleeding. The chance is higher if you: are age 60 or older, have had stomach ulcers or bleeding problems, take a blood thinner (anticoagulant) or steroid drug, take other drugs containing NSAIDs (aspirin, ibuprofen, naproxen, or others), have 3 or more alcoholic drinks every day while using this product, take more or for a longer time than directed - DO NOT USE
Close
Do not use if you have ever had an allergic reaction to any other pain reliever/fever reducer, right before or after heart surgery. - ASK DOCTOR
Ask a doctor before use if stomach bleeding warning applies to you; you have a history of stomach problems such as heartburn; you have a high blood pressure, heart disease, liver cirrhosis, or kidney disease; you are taking a diuretic
Close - ASK DOCTOR/PHARMACIST
Ask a doctor before use if you are:
Close
taking any other drug containing NSAID (prescription or nonprescription); taking aspirin for heart attack or stroke, because Ibuprofen may decrease this benefit of aspirin; taking any other drug - WHEN USING
- STOP USE
Stop use and ask a doctor If:
Close
you experience any of the following signs of stomach bleeding; feel faint; vomit blood; have bloody or black stools; have stomach pain that does get better; pain gets worse or lasts more than 10 days; fever gets worse or lasts more than 3 days; redness or swelling is present in the painful area; any new symptoms appear - PREGNANCY OR BREAST FEEDING
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use ibuprofen during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Close - KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Close - DOSAGE & ADMINISTRATION
Directions:
Close
do not use more than directed; the smallest effective dose should be used; do not take longer than 10 days, unless directed by a doctor.
Adults and Children (12 years and older): Take 1 tablet every 4 to 6 hours while symptoms persist. If pain or fever does not respond to 1 tablet, 2 tablets may be used. Do not exceed 6 tablets in 24 hours, unless directed by a doctor.
Children under 12 years: Do not give to children under 12 years of age. - STORAGE AND HANDLING
Close
Other information: Store at controlled room temperature; avoid excessive heat 40 degree Celsius (104 degree Fahrenheit); tamper evident sealed packets; do not use any opened or torn packets - INACTIVE INGREDIENT
Inactive ingredients: cellulose, corn starch, fumed silica gel, hypromellose, lactose, magnesium stearate, polydextrose, polyethylene glycol, povidone, silica, sodium starch glycolate, stearic acid, titanium dioxide, triacetin. Close
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
Active Ingredient (in each tablet) Purpose
Close
Acetaminophen 325 mg ............. Analgesic/antipyretic - PURPOSE
Uses
Close
temporary relief of minor aches and pains associated with:
common cold; headache; toothache; muscular aches; backache; arthritis; menstrual cramps; and reduction of fever - WARNINGS
Warnings:
Close
Liver warning: This product contains acetaminophen.
Severe liver damage may occur if: adult takes more than 12 tablets in 24 hours, which is the maximum daily amount; child takes more than 5 doses in 24 hours; taken with other drugs containing acetaminophen; adult has 3 or more alcoholic drinks every day while using this product - DO NOT USE
Do not use: with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist; for more than 10 days for pain unless directed by a doctor; for more than 3 days for fever unless directed by a doctor
Close - ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- STOP USE
Stop use and ask a doctor if: symptoms do not improve; pain gets worse or lasts for more than 10 days; fever gets worse or lasts for more than 3 days; new symptoms occur; redness or swelling is present; a rare sensitivity reaction occurs
Close - PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. In case of accidental overdose, contact a doctor or Poison Control Center immediately. Prompt
Close
medical attention is critical for adults as well as for children even if
you do not notice any signs or symptoms. Do not exceed recommended dosage - DOSAGE & ADMINISTRATION
Directions
Close
Adults and Children Take 2 tablets every 4 to 6 hours as
12 years of age needed. Do not take more than 12 tablets
or older in 24 hours.
Children 6-11 years Take 1 tablet every 4 to 6 hours as
of age needed. Do not take more than 5
tablets in 24 hours.
Children under 6 Do not use this regular strength product.
years of age This will provide more than the
recommended dose (overdose) and could
cause serious health problems. - STORAGE AND HANDLING
- GENERAL PRECAUTIONS
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- ACTIVE INGREDIENT
Active Ingredient (in each tablet) Purpose
Close
Aspirin (NSAID*) 325 mg............................... Pain Reliever / fever reducer
*nonsteroidal anti-inflammatory drug - PURPOSE
Uses Temporarily relieves minor aches and pains associated with:
Close
headache ; muscular aches ; minor arthritis pain ; backache ; common cold ; toothache ; menstrual cramps ; Temporarily reduces fever - WARNINGS
Warnings
Close
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox of flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include: hives, skin reddening, facial swelling, rash, asthma (wheezing), blisters, shock, If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
are age 60 or older; have had stomach ulcers or bleeding problems; take a blood thinner (anticoagulant) or steroid drug; take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others); have 3 or more alcoholic drinks every day while using this product; take more or for a longer time than directed - DO NOT USE
Do not use: if you have ever had an allergic reaction to any other pain reliever/ fever reducer; right before or after heart surgery; if you are taking a prescription drug for gout, diabetes or arthritis
Close - ASK DOCTOR
Ask a doctor before use if: stomach bleeding warning applies to you; you have a history of stomach problems such as heartburn; you have high blood pressure, heart disease, liver cirrhosis, or kidney disease; you are taking a diuretic
Close - ASK DOCTOR/PHARMACIST
Ask a doctor or pharmacist before use if you are:
Close
under a doctor's care for any serious condition; taking any other drug - WHEN USING
- STOP USE
Close
Stop use and ask a doctor if
you experience any of the following signs of stomach bleeding:
feel faint; vomit blood; have bloody or black stools; have stomach
pain that does not get better; pain gets worse or lasts more than 10 days; fever gets worse or lasts more than 3 days; you have difficulty swallowing; if ringing in the ears or loss of hearing occurs; redness or swelling is present in the painful areas; any new symptoms appear - PREGNANCY OR BREAST FEEDING
If pregnant or breast-feeding, ask a health professional before use. It is especially important to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Close - KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. In case of overdose, get medical help or
Close
contact a Poison Control Center right away. - WHEN USING
Directions
Close
do not use more than directed
the smallest effective dose should be used
drink a full glass of water with each dose
do not take longer than 10 days, unless directed by a doctor - DOSAGE & ADMINISTRATION
Adults and children: (12 years and older) Take 1 or 2 tablets with
Close
water every 4 hours as needed. Do not take more than 12 tablets in 24
hours, or as directed by a doctor.
Children under 12 years: Do not give to children under 12 years of age. - STORAGE AND HANDLING
Store at 59 - 86 degree Fahrenheit (15 - 30 degree Celsius); avoid
Close
excessive heat and humidity; tamper evident sealed packets;
Do not use any opened or torn packets - INACTIVE INGREDIENT
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
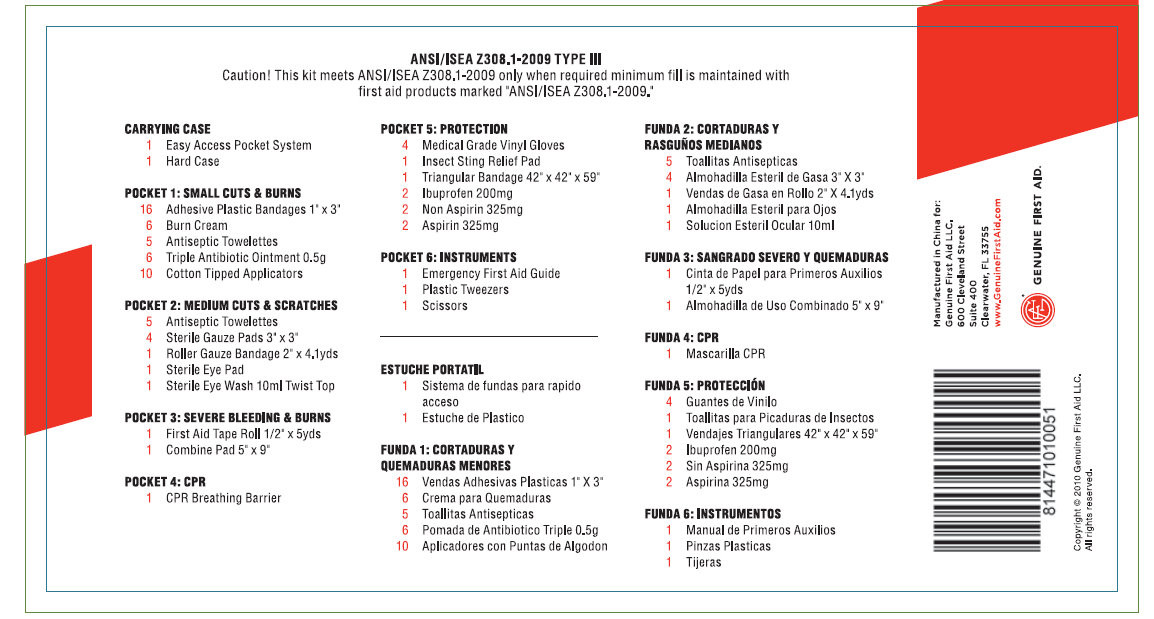
ANSI/ISEA Z308.1-2009 TYPE III
Caution! This Kit meets ANSI/ISEA Z308.1-2009 only when required minimum fill is maintained with first aid products marked "ANSI/ISEA Z308.1-2009."
CARRYING CASE
1 Easy Access Pocket
System
1 Hard CasePOCKET1: SMALL CUTS AND BURNS
16 Adhesive Plastic Bandages 1"x3"
1 Burn Cream
5 Antiseptic Towelettes
6 Triple Antibiotic Ointment 0.5gr
10 Cotton Tipped Applicators
POCKET 2: MEDIUM CUTS AND SCRATCHES
5 Antiseptic Towelettes
4 Sterile Gauze Pad 3"x3"
1 Roller Gauze Bandage 2"X4.1yds
1 Sterile Eye Pads
1 Sterile Eye Wash 10ml Twist Top
POCKET 3: SEVERE BLEEDING AND BURNS
1 First Aid Tape Roll 1/2"x5 yds.
1 Combine Pad 5"X9"POCKET 4: CPR
1 CPR Breathing Barrier
POCKET 5: PROTECTION
2 Medical Grade Vinyl Gloves
1 Insect Sting Relief Pads
1 Triangular Bandage 42"x42"x59"
2 Ibuprofen 200mg
2Non Aspirin 325mg
2 Aspirin 325mgPOCKET 6: INSTRUMENTS
1 Emergency First Aid Guide
1 Plastic Tweezers
1 Scissors
Manufactured in China for:
Genuine First Aid LLC.
600 Cleveland Street
Suite 400
Clearwater FL 33755
www.GenuineFirstAid.comGENUINE FIRST AID
Copyright c 2010 Genuine First Aid LLC. All rights reserved.
Close - INGREDIENTS AND APPEARANCE
10 PERSON ANSI
benzalkonium chloride, lidocaine, bacitracin zinc, neomycin sulfate, polymyxin b sulfate, water, benzocaine, alcohol, ibuprofen, acetaminophen, aspirin kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52124-0111 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0111-1 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 PACKAGE 5.4 g Part 2 10 PACKAGE 8 mL Part 3 6 TUBE 3 g Part 4 1 BOTTLE 10 mL Part 5 1 PACKAGE 0.5 mL Part 6 1 PACKAGE 2 Part 7 1 PACKAGE 2 Part 8 1 PACKAGE 2 Part 1 of 8 GENUINE FIRST AID BURN ANTISEPTIC PAIN RELIEF WITH ALOE
benzalkonium chloride, lidocaine creamProduct Information Item Code (Source) NDC:52124-0004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 0.5 in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0004-1 0.9 g in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part345 04/24/2010 Part 2 of 8 ANTISEPTIC TOWELETTE
benzalkonium chloride liquidProduct Information Item Code (Source) NDC:52124-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.40 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0001-1 0.8 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 04/24/2010 Part 3 of 8 GENUINE TRIPLE ANTIBIOTIC
bacitracin zinc,neomycin sulfate,polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC:52124-0003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN ZINC 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B SULFATE 5000 [iU] in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0003-1 0.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 04/24/2010 Part 4 of 8 STERILE ISOTONIC BUFFERED GENUINE EYEWASH
water liquidProduct Information Item Code (Source) NDC:52124-0005 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) EDETATE DISODIUM (UNII: 7FLD91C86K) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0005-1 10 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 04/24/2010 Part 5 of 8 INSECT STING RELIEF PAD
benzocaine,alcohol liquidProduct Information Item Code (Source) NDC:52124-0008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 6 mL in 100 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 60 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0008-1 0.5 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 04/24/2010 Part 6 of 8 IBUPROFEN
ibuprofen tabletProduct Information Item Code (Source) NDC:52124-0009 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength POWDERED CELLULOSE (UNII: SMD1X3XO9M) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE (UNII: 3NXW29V3WO) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white (White) Score no score Shape ROUND Size 10mm Flavor Imprint Code 44;352 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0009-1 2 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075010 04/24/2010 Part 7 of 8 NON-ASPIRIN
acetaminophen tabletProduct Information Item Code (Source) NDC:52124-0010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) STEARIC ACID (UNII: 4ELV7Z65AP) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white (WHITE) Score no score Shape ROUND Size 11mm Flavor Imprint Code AZ;234 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0010-1 2 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part343 04/24/2010 Part 8 of 8 ASPIRIN
aspirin tabletProduct Information Item Code (Source) NDC:52124-0011 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (White) Score no score Shape ROUND Size 11mm Flavor Imprint Code 44;157;ASPIRIN Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52124-0011-1 2 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part343 04/24/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 04/24/2010 Labeler - Genuine First Aid LLC (619609857) CloseEstablishment Name Address ID/FEI Business Operations GFA Production ( Xiamen) Co., Ltd 421256261 manufacture